Does Hbr Have Dipole Dipole Forces
Evidently with its extra mass it has much stronger. That HBr has a higher boiling point proves that it is has stronger intermolecular attractions despite its lesser dipole moment.
HCl has stronger London dispersion forces d.
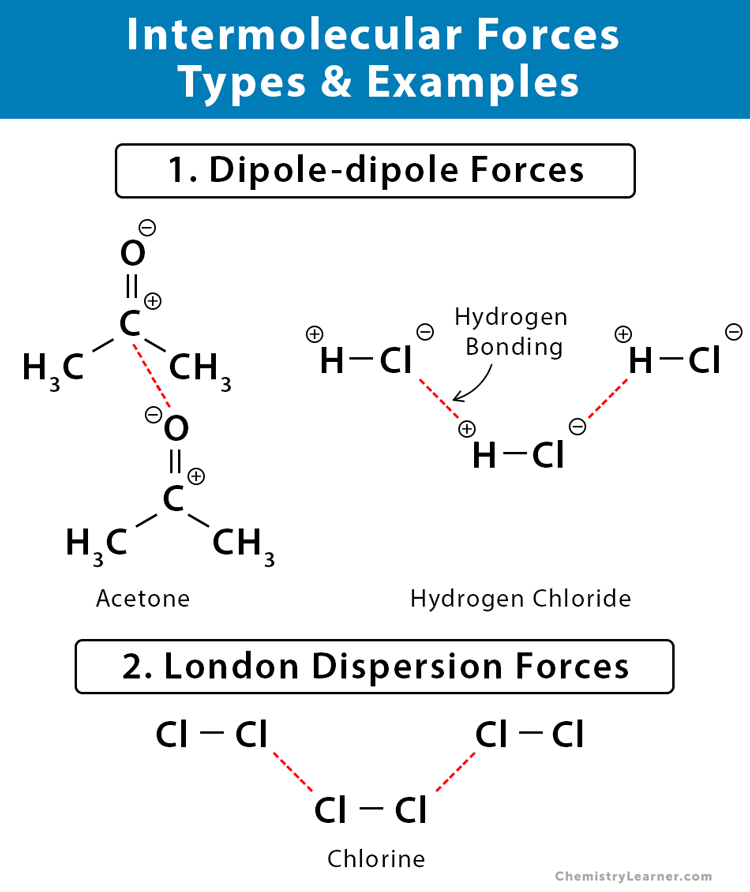
. HBr is more polar. Both molecules have hydrogen bonding. HCl has stronger intermolecular forces.

P Block Elements Class 12 Notes Vidyakul In 2022 Chemistry Notes Dissociation Chemistry

Intermolecular Forces Definition Types And Examples
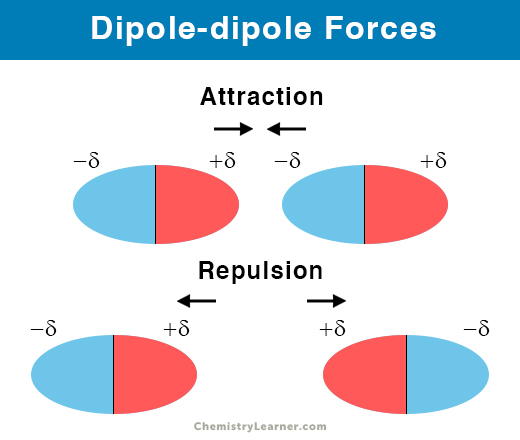
Dipole Dipole Forces Definition And Examples

Weak Intermolecular Bonding Intermolecular Force Chemistry Science

No comments for "Does Hbr Have Dipole Dipole Forces"
Post a Comment